Abstract
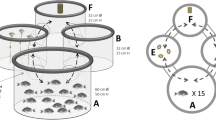
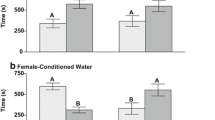
Nurse sharks (Ginglymostoma cirratum, Ginglymostomatidae) are one of the most exhibited shark species in aquariums worldwide. However, in these locations, their reproductive success rate is frequently low. Considering that there is probably an olfactory mediation for nurse shark reproduction, and that environmental enrichment has a potentially positive effect on reproduction of captive animals, the present study aimed to evaluate whether the use of olfactory enrichment would stimulate olfactory-driven behaviors and consequently generate effects in the exhibition of reproductive behaviors by the species both in the short and long term. The study was divided into three phases (control, short-term enrichment and long-term enrichment), was conducted at two institutions with distinct husbandry and used aqueous extracts of basil leaves as olfactory enrichment. Eleven individuals were selected through focal sampling for behavioral observations with instantaneous recording, the total of 120 h of observation were divided into active and inactive periods. Results showed that basil-based olfactory enrichment was effective in increasing the occurrence of reproductive behaviors in both female and male nurse sharks. Additionally, the long-term results showed higher effects in males, which strengthen the theory of olfactory mediation of reproductive behavior in the species as individuals became aware of the sensory cues in the environment and performed more olfactory-driven reproductive behaviors. It is important to note that enrichment effects are different between sexes and therefore close monitoring and scheduling are essential to avoid over-stimulation or habituation to the enrichment.



Similar content being viewed by others
Notes
Projeto Tamar, personal communication
References
AES (2008) The 2008 AES international captive elasmobranch census
Ahlbeck Bergendahl I, Salvanes AGV, Braithwaite VA (2016) Determining the effects of duration and recency of exposure to environmental enrichment. Appl Anim Behav Sci 176:163–169. https://doi.org/10.1016/j.applanim.2015.11.002
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Amo L (2017) The role of olfaction in mate selection and reproductive behaviour. In: Olfaction in animal behaviour and welfare. CABI, Wallingford, pp 85–101. https://doi.org/10.1079/9781786391599.0085
Anderson C, Arun AS, Jensen P (2010) Habituation to environmental enrichment in captive sloth bears-effect on stereotypies. Zoo Biol 29:705–714. https://doi.org/10.1002/zoo.20301
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J stat Softw 67. https://doi.org/10.18637/jss.v067.i01
Beach FA (1976) Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav 7:105–138. https://doi.org/10.1016/0018-506X(76)90008-8
Carlstead K, Shepherdson D (1994) Effects of environmental enrichment on reproduction. Zoo Biol 13:447–458. https://doi.org/10.1002/zoo.1430130507
Carrier JC, Murru FL, Walsh MT, Pratt HL (2003) Assessing reproductive potential and gestation in nurse sharks (Ginglymostoma cirratum) using ultrasonography and endoscopy: an example of bridging the gap between field research and captive studies. Zoo Biol 22:179–187. https://doi.org/10.1002/zoo.10088
Carrier JC, Pratt HL, Martin LK (1994) Group reproductive behaviors in free-living nurse sharks, Ginglymostoma cirratum. Copeia 1994:646. https://doi.org/10.2307/1447180
Castro ALF, Rosa RS (2005) Use of natural marks on population estimates of the nurse shark, Ginglymostoma cirratum, at Atol das Rocas biological reserve, Brazil. Environ Biol Fish 72:213–221. https://doi.org/10.1007/s10641-004-1479-7
Ceballos G, Ehrlich PR, Barnosky AD, García A, Pringle RM, Palmer TM (2015) Accelerated modern human–induced species losses: entering the sixth mass extinction. Sci Adv 1:e1400253. https://doi.org/10.1126/sciadv.1400253
Clark F, King AJ (2008) A critical review of zoo-based olfactory enrichment. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD (eds) Chemical signals in vertebrates 11. Springer New York, New York, NY, pp 391–398. https://doi.org/10.1007/978-0-387-73945-8_37
Colbachini H, Pizzutto CS, Jorge-Neto PN, Gutierrez RC, Gadig OBF (2020) Body movement as an indicator of proceptive behavior in nurse sharks (Ginglymostoma cirratum). Environ Biol Fish 103:1257–1263. https://doi.org/10.1007/s10641-020-01018-y
Compagno LJV (2001) Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Volume 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes), FAO species catalogue for fishery purposes. FAO, Rome
Conway WG (2011) Buying time for wild animals with zoos. Zoo Biol 30:1–8. https://doi.org/10.1002/zoo.20352
Crawley MJ (2013) The R book, 2nd ed. John Wiley & Sons, Ltd, Chichester, UK https://doi.org/10.1002/9781118448908
Crofton EJ, Zhang Y, Green TA (2015) Inoculation stress hypothesis of environmental enrichment. Neurosci Biobehav Rev 49:19–31. https://doi.org/10.1016/j.neubiorev.2014.11.017
Crooks N, Waring CP (2013) A study into the sexual dimorphisms of the Ampullae of Lorenzini in the lesser-spotted catshark, Scyliorhinus canicula (Linnaeus, 1758). Environ Biol Fish 96:585–590. https://doi.org/10.1007/s10641-012-0048-8
da Silva R, Pearce-Kelly P, Zimmerman B, Knott M, Foden W, Conde DA (2019) Assessing the conservation potential of fish and corals in aquariums globally. J Nat Conserv 48:1–11. https://doi.org/10.1016/j.jnc.2018.12.001
de Azevedo CS, Cipreste CF, Young RJ (2007) Environmental enrichment: A GAP analysis. Appl Anim Behav Sci 102:329–343. https://doi.org/10.1016/j.applanim.2006.05.034
Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Carlson JK, Davidson LN, Fordham SV, Francis MP, Pollock CM, Simpfendorfer CA, Burgess GH, Carpenter KE, Compagno LJ, Ebert DA, Gibson C, Heupel MR, Livingstone SR, Sanciangco JC, Stevens JD, Valenti S, White WT (2014) Extinction risk and conservation of the world’s sharks and rays. Elife 3. https://doi.org/10.7554/eLife.00590
Gardiner JM, Hueter RE, Maruska KP, Sisneros JA, Casper BM, Mann DA, Demski LS (2012) Sensory physiology and behavior of elasmobranchs. In: Carrier JC, Musick JA, Heithaus MR (eds) Biology of sharks and their relatives. CRC Press, Boca Raton, pp 365–418. https://doi.org/10.1201/b11867-20
Garla RC, Garcia J, Veras LB, Lopes NP (2009) Fernando de Noronha as an insular nursery area for lemon sharks, Negaprion brevirostris, and nurse sharks, Ginglymostoma cirratum, in the equatorial western Atlantic Ocean. Mar Biodivers Rec 2:e109. https://doi.org/10.1017/S1755267209000670
Greenway E, Jones KS, Cooke GM (2016) Environmental enrichment in captive juvenile thornback rays, Raja clavata (Linnaeus 1758). Appl Anim Behav Sci 182:86–93. https://doi.org/10.1016/j.applanim.2016.06.008
Henningsen AD, Smale M, Garner R, Kinnunen N (2004a) Reproduction, embryonic development, and reproductive physiology of elasmobranchs, in: Smith M, Warmolts D, Thoney D, Hueter R (Eds.) The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives. Ohio Biological Survey, Inc. 2004, Columbus, Ohio, pp 227–236
Henningsen AD, Smale MJ, Gordon I, Garner R, Marin-Osorno R, Kinnunen N (2004b) Captive breeding and sexual conflict in elasmobranchs, in: Smith M, Warmolts D, Thoney D, Hueter R (Eds.) The Elasmobranch Husbandry Manual: Captive Care of Sharks, Rays and Their Relatives. Ohio Biological Survey, Inc. 2004, Columbus, Ohio, pp. 237–248
ICMBio (2018) Livro Vermelho da Fauna Brasileira Ameaçada de Extinção: Volume VI - Peixes. ICMBio/MMA, Brasília
Janse M, Zimmerman B, Geerlings L, Brown C (2017) Sustainable species management of the elasmobranch populations within European aquariums: a conservation challenge. J Zoo Aquarium Res 5:172–181. https://doi.org/10.19227/jzar.v5i4.313
Janssen JD, Kidd A, Ferreira A, Snowden S (2017) Training and conditioning of elasmobranchs in aquaria, in: Smith M, Warmolts D, Thoney D, Hueter R, Murray M, Ezcurra J (Eds.) The elasmobranch husbandry manual II: recent advances in the Care of Sharks, Rays and Their Relatives. Ohio Biological Survey, Inc., Columbus, pp. 209–221
Johnson RH, Nelson DR (1978) Copulation and possible olfaction-mediated pair formation in two species of Carcharhinid sharks. Copeia 1978:539. https://doi.org/10.2307/1443626
Khaki A, Azad FF, Nouri M, Khaki AA (2011) Effects of basil, ocimum basilicum on spermatogenesis in rats. J Med Plant Res 5:4601–4604. https://doi.org/10.5897/JMPR.9000511
Khaki A, Khaki AA, Ezzatzadeh A, A-Ashteani H (2013) Effect of Ocimum basilicum on ovary tissue histopathology after exposure to electromagnetic fields (EMF) in rats. African J Pharm Pharmacol 7:1703–1706. https://doi.org/10.5897/AJPP12.1073
Klimley AP (1980) Observations of courtship and copulation in the nurse shark, Ginglymostoma cirratum. Copeia 1980:878. https://doi.org/10.2307/1444471
Koop JH (2005) Reproduction of captive Raja spp. in the Dolfinarium Harderwijk. J Mar Biol Assoc United Kingdom 85:1201–1202. https://doi.org/10.1017/S0025315405012312
Kuczaj S, Lacinak T, Fad O, Trone M, Solangi M, Ramos J (2002) Keeping environmental enrichment enriching. Int J Comp Psychol 15:127–137
Kuenen M (2000) A log of captive births by an Atlantic nurse shark, “Sarah”. Drum Croak 31:22–24
Lee KA, Huveneers C, Peddemors V, Boomer A, Harcourt RG (2015) Born to be free? Assessing the viability of releasing captive-bred wobbegongs to restock depleted populations. Front Mar Sci 2:1–14. https://doi.org/10.3389/fmars.2015.00018
Lehner PN (1998) Handbook of ethological methods, 2nd edn. Cambridge University Press, New York
Luer CA, Gilbert PW (1985) Mating behavior, egg deposition, incubation period, and hatching in the clearnose skate, Raja eglanteria. Environ Biol Fish 13:161–171. https://doi.org/10.1007/BF00000926
Marin-Osorno R, Ezcurra JM, O’Sullivan JB (2017) Husbandry of the Tiger shark, Galeocerdo cuvier, at the Acuario de Veracruz, México, in: Smith M, Warmolts D, Thoney D, Hueter R, Murray M, Ezcurra J (Eds.) The elasmobranch husbandry manual II: recent advances in the Care of Sharks, Rays and Their Relatives. Ohio Biological Survey, Inc., Columbus, pp. 23–32
McFarlane GA, Wydoski RS, Prince ED (1990) External tags and marks. Am Fish Soc Symp 7:9–29
Meagher RK, Ahloy Dallaire J, Campbell DLM, Ross M, Møller SH, Hansen SW, Díez-León M, Palme R, Mason GJ (2014) Benefits of a ball and chain: simple environmental enrichments improve welfare and reproductive success in farmed american mink (Neovison vison). PLoS One 9:e110589. https://doi.org/10.1371/journal.pone.0110589
Moreira N, Brown JL, Moraes W, Swanson WF, Monteiro-Filho ELA (2007) Effect of housing and environmental enrichment on adrenocortical activity, behavior and reproductive cyclicity in the female tigrina (Leopardus tigrinus) and margay (Leopardus wiedii). Zoo Biol 26:441–460. https://doi.org/10.1002/zoo.20139
Näslund J, Johnsson JI (2016) Environmental enrichment for fish in captive environments: effects of physical structures and substrates. Fish Fish 17:1–30. https://doi.org/10.1111/faf.12088
Newberry RC (1995) Environmental enrichment: increasing the biological relevance of captive environments. Appl Anim Behav Sci 44:229–243
Nielsen BL, Jezierski T, Bolhuis JE, Amo L, Rosell F, Oostindjer M, Christensen JW, McKeegan D, Wells DL, Hepper P (2015) Olfaction: an overlooked sensory modality in applied ethology and animal welfare Front Vet Sci 2. https://doi.org/10.3389/fvets.2015.00069
Penning M, Reid GM, Koldewey H, Dick G, Andrews B, Arai K, Garratt P, Gendron S, Lange J, Tanner K, Tonge S, Van den Sande P, Warmolts D, Gibson C (2009). Turning the tide: A global aquarium strategy for conservation and sustainability. World Association of Zoos and Aquariums (WAZA), Bern
Pine W, Hightower J, Coggins L, Lauretta M, Pollock K (2012) Design and analysis of tagging studies. In: Zale AV, Parrish DL, Sutton TM (eds) Fisheries Techniques. American Fisheries Society, Bethesda, pp 521–572
Pratt HL Jr, Carrier JC (2001) A review of elasmobranch reproductive behavior with a case study on the nurse shark, Ginglymostoma cirratum. Environ Biol Fish 60:157–188. https://doi.org/10.1023/A:1007656126281
R Development Core Team (2011) R: A language and environment for statistical computing. the R Foundation for Statistical Computing., Viena
Rosa RS, Castro ALF, Furtado M, Monzini J, Grubbs RD (2006) Ginglymostoma cirratum(Western Atlantic subpopulation). The IUCN red list of threatened species 2006: e.T60224A12327471 [WWW document]. https://doi.org/10.2305/IUCN.UK.2006.RLTS.T60224A12327471.en
Samuelson MM, Lauderdale LK, Pulis K, Solangi M, Hoffland T, Lyn H (2017) Olfactory enrichment in California Sea lions (Zalophus californianus): an effective tool for captive welfare? J Appl Anim Welf Sci 20:75–85. https://doi.org/10.1080/10888705.2016.1246362
Shepherdson D (1994) The role of environmental enrichment in the captive breeding and reintroduction of endangered species. In: Olney PJS, Mace GM, Feistner ATC (eds) Creative conservation: interactive management of wild and captive animals. Chapman & Hall, London, pp 167–177
Tarou LR, Bashaw MJ (2007) Maximizing the effectiveness of environmental enrichment: suggestions from the experimental analysis of behavior. Appl Anim Behav Sci 102:189–204. https://doi.org/10.1016/j.applanim.2006.05.026
Wafer LN, Jensen VB, Whitney JC, Gomez TH, Flores R, Goodwin BS (2016) Effects of environmental enrichment on the fertility and fecundity of zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 55:291–294
Waghray S (1985) Olfactory organ and its sexual dimorphism in the electric ray, Narcine timlei (day). Indian J Fish 32:148–151
Wells DL (2009) Sensory stimulation as environmental enrichment for captive animals: a review. Appl Anim Behav Sci 118:1–11. https://doi.org/10.1016/j.applanim.2009.01.002
Wells DL, Egli JM (2004) The influence of olfactory enrichment on the behaviour of captive black-footed cats, Felis nigripes. Appl Anim Behav Sci 85:107–119. https://doi.org/10.1016/j.applanim.2003.08.013
Whitney NM, Lear KO, Gaskins LC, Gleiss AC (2016) The effects of temperature and swimming speed on the metabolic rate of the nurse shark (Ginglymostoma cirratum, Bonaterre). J Exp Mar Bio Ecol 477:40–46. https://doi.org/10.1016/j.jembe.2015.12.009
Yano K, Sato F, Takahashi T (1999) Observations of mating behavior of the manta ray,Manta birostris, at the ogasawara islands. Japan Ichthyol Res 46:289–296. https://doi.org/10.1007/BF02678515
Young RJ (2003) Environmental enrichment for captive animals, 1st edition. Ed. Blackwell science ltd, Universities Federation for Animal Welfare (UFAW), Oxford, UK. https://doi.org/10.1002/9780470751046
Zar JH (2010) Biostatistical analysis, 5th Editio. ed. Pearson Prentice Hall, Upper Saddle River
Brasil (2018) Acordo de Cooperação Técnica n° 3202386. Processo n° 02070.003869/2018-45 from June 05, 2018. Diário Oficial da União, Brasília, DF, ed 106, sec 3, pp. 108
Acknowledgments
The authors thank the São Paulo Aquarium and the TAMAR Project staff for maintaining the sharks used in the present study and allowing observations out of working hours. They also thank all the contributors to the work, especially M. Miranda, B. Ogata, C. S. de Azevedo, C. Colbachini and W. Vilegas.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
H Colbachini, LM de Souza Mesquita and OBF Gadig performed the experiments. H Colbachini, CS Pizzutto, LM de Souza Mesquita and OBF Gadig analyzed and interpreted the data. H Colbachini and CS Pizzutto wrote the manuscript. H Colbachini, CS Pizzutto, LM de Souza Mesquita and OBF Gadig critically revised the manuscript. All authors approved the manuscript for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
The use and care of experimental animals obeyed the Brazilian laws, guidelines and policies on animal welfare, according to IBAMA (IN 07/2015) and by the institutions technical staff. As no invasive method was applied, no further ethical approval was required.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Colbachini, H., Pizzutto, C.S., de Souza Mesquita, L.M. et al. Environmental enrichment effects on the reproductive behavior of captive nurse sharks Ginglymostoma cirratum. Environ Biol Fish 104, 471–488 (2021). https://doi.org/10.1007/s10641-021-01087-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-021-01087-7